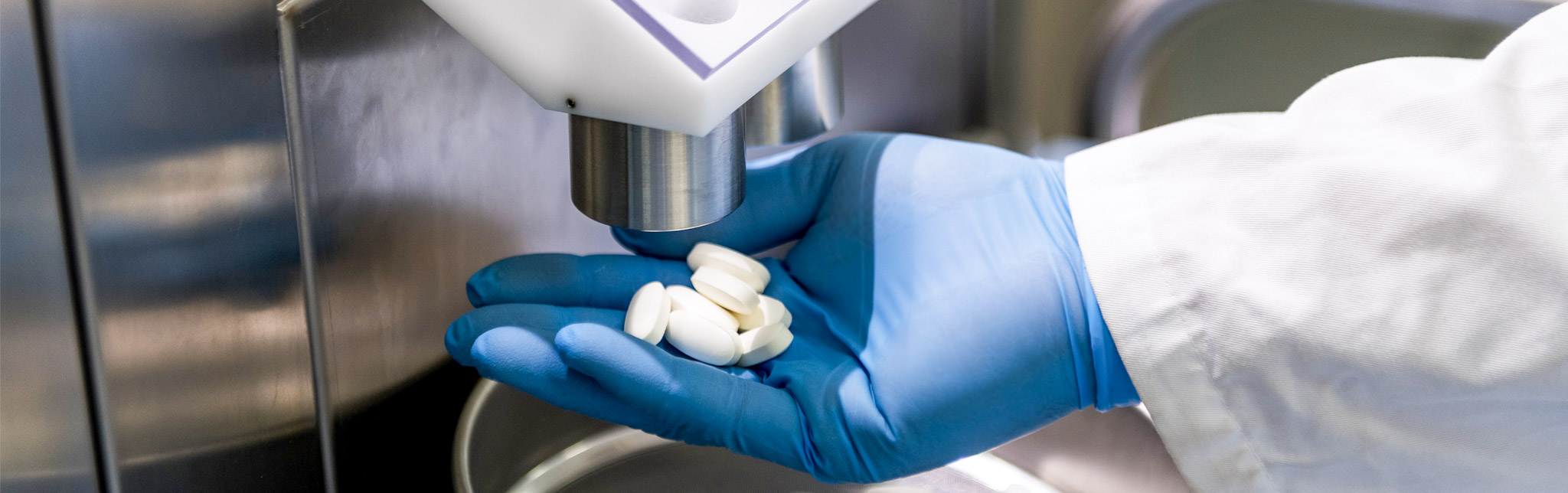
Cosmo has developed over the years an important manufacturing experience.
We provide services related to the production of pharmaceutical products as: assessment of manufacturing processes, technology transfer, evaluation of pharmaceutical product stability, testing and release to the market and drafting of the necessary documentation for pharmaceutical products’ registration.
We are authorized by many regulation authorities including AIFA, FDA, PMDA, ANVISA, RUSSIA Health Authority to produce medicines for the European and U.S. markets as well as for many other markets in EMEA, APAC and US. The plant’s quality system is certified in accordance with the ISO 13485 standards and is focused on continuous improvement and customer satisfaction.
Our productive plant, based in Lainate, covers an area of 18000 m2 and is devoted to the production of drugs and the preparation of clinical batches.
Our plant can produce solid, semi-solid and liquid oral medicines on the group’s behalf for Italian and international pharmaceutical companies; we have over 30 years of experience in tablet and film coating tablets development and production with seven production departments equipped for wet and dry granulation, tableting and film coating.
Cosmo is equipped with serialization and aggregation systems to satisfy the entire pharmaceutical product traceability covering different requirements around the world.
The quality department
The quality department includes the quality control labs and the validation and regulatory affairs units and has the responsibility of ensuring the quality of the pharmaceutical products through specific standard operating procedures (SOPs), which ensure compliance in the application of current good manufacturing practices (GMPs).
The cutting-edge analytical instrumentation, validated in accordance with the regulations in force (EU GMP Annex 11) (FDA 21 CFR Part 11), guarantees the accuracy of the analytical results and the integrity of the electronic data.
Business continuity is assured thanks to availability of backup facility and available additional production capability.