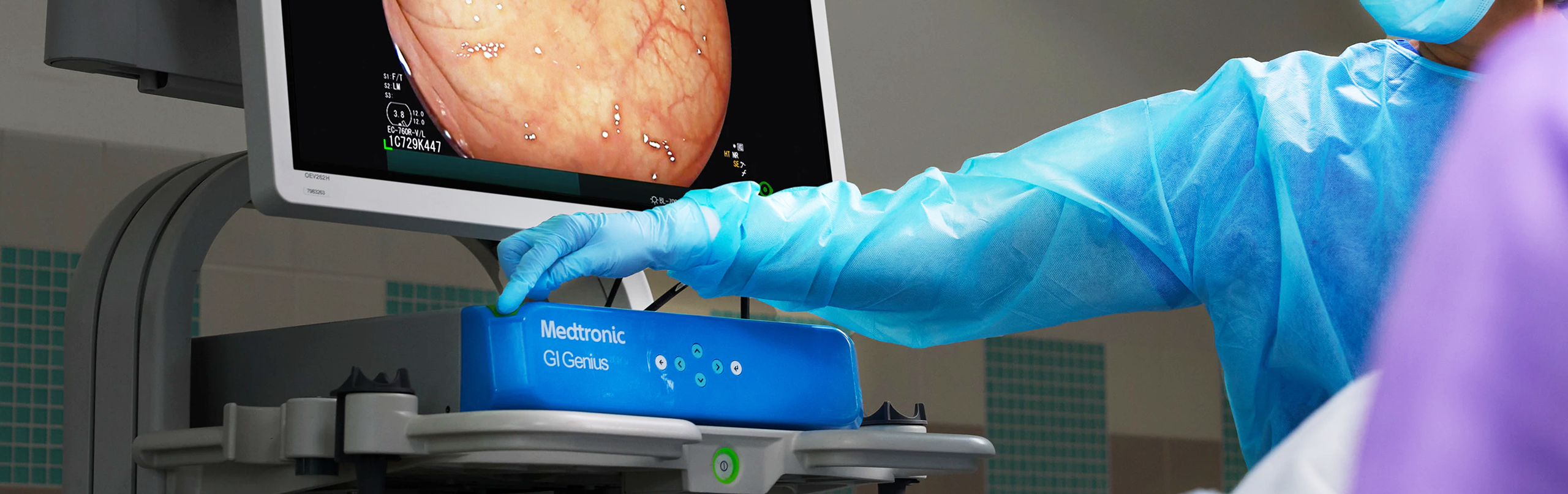
GI Genius™ is the first-to-market, deep learning, computer-assisted system that uses artificial intelligence in real time to detect colorectal lesions such as polyps and adenomas of different shapes, sizes and morphologies in real time. The device can be seamlessly integrated and is compatible with all major brands of endoscopic processors.
GI Genius™ increases ADR1 (adenoma detection rate) and decreases AMR2 (adenoma miss rate) as compared to standard of care. In an investigator-initiated prospective clinical study of GI Genius, the device was more effective than the standard of care, i.e. high-definition white-light colonoscopy (control group): ADR was significantly higher in the GI Genius™ group than in the control group (54.8% versus 40.4%, respectively; absolute difference: +14.4%; relative difference: +35.6%) APC was 50.7% higher in the GI Genius™ group than in the control group (1.07±1.54 versus 0.71±1.20 respectively)1.
The first U.S. Trial using GI genius™ intelligent endoscopy module showed 50% reduction in missed colorectal polyps with artificial intelligence (AI) technology versus the standard of care, i.e. high-definition white-light colonoscopy (control group): polyp miss rate (PMR) was 16.9% in the GI Genius group and 31.1 % in the control group (p<0.001) as well as adenoma miss rate (AMR) was 15.5% in GI Genius arm and 32.4% control group (p < 0.001). Importantly, use of GI Genius was associated with a notable decrease in the miss rate for those lesions most difficult to see with the standard of care, i.e. flat lesions (flat adenoma miss rate with GI Genius: 16.8% vs 45.8% control; p<0.001)2.
The device is already approved in Europe (it is a CE-marked medical device) and in April 2021 GI Genius™ was approved by the FDA. GI Genius™ is also approved in Australia, Canada, Switzerland, Singapore, Israel, UAE and U.K.
To know more about GI Genius™ and our vision for Artificial Intelligence, visit our dedicated website: Cosmo IMD

Global AI partnership with Medtronic
The device is developed by Cosmo Intelligent Medical Devices, a fully owned subsidiary of Cosmo Pharmaceuticals, and it is marketed worldwide by Medtronic, the global leader in medical technology.
In March 2023, Cosmo Intelligent Medical Devices expanded the cooperation with Medtronic to accelerate the development of Artificial Intelligence (AI) in the healthcare system and bring new AI-based solutions into patient care.
Gi Genius won the Artificial Intelligence Innovation in 2023 AI Breakthrough Awards Program
____________
1Repici et al. 2020. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 159(2):512-520.e7. https://doi.org/10.1053/j.gastro.2020.04.062
2Wallace et al. 2022 .Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology 163(1):295-304.e5. https://doi.org/10.1053/j.gastro.2022.03.007